Vaccination and immunity
Vaccination is a process involving the introduction of antigens from a pathogen in order to stimulate the immune system to develop adaptive immunity to it.
The material that is introduced is called a
vaccine, and it is normally produced and stored under strictly controlled conditions of hygiene and scientific provenance of the source pathogen ( strain, virulence etc).
Vaccination can prevent against disease or at least reduce the seriousness of it.
Vaccination may need to be carried out several times - as 'boosters' - in order to raise the antibody level (secondary responses -
see above), or if memory cells become depleted over time.
Types of vaccine
There are several sorts of material used to provide the antigens which are the active component of a vaccine:
| Source material |
Example |
| 'live' viruses (which are generally weaker or 'attenuated strains' of the virus) |
Measles, mumps, rubella (MMR combined vaccine) |
| inactivated or killed organisms (viruses or bacteria) |
Influenza
BCG Vaccine (TB vaccine)
|
| inactivated toxins (from bacteria) |
Tetanus (Lockjaw)
|
| segments of the outer covering of the pathogen (subunit, recombinant, polysaccharide, and conjugate vaccines)
|
Human papillomavirus (HPV) |
| mRNA coding for segments of viral protein (spike protein)
|
Coronavirus (Covid-19) |
Sources of antigens
Bacteria can be cultured fairly easily using standard laboratory techniques: they grow on sterilised media in flasks, in incubators.
On the other hand, viruses need to grow inside living cells, so there needs to be a supply of these before they can be infected with active/'living' viruses.
Live whole organisms (animals) are not used in vaccine production, but cultures of cells ('cell lines') - usually from animals, or occasionally of human origin, are often used.
There are two basic levels of supply
-
master/reference cultures (originating in laboratories) - organisms/viruses proven to control the disease in question, but may have been weakened by growing at suboptimal temperature/in presence of 'attenuating agent' e.g. formalin
-
production cultures - derived from the above, but not reused indefinitely, mainly for hygiene reasons.
There is a broad similarity with food or drink (cheese, yoghurt, wine, beer) production techniques which use starter cultures.
After incubation, the bacteria or viruses are extracted from the surrounding medium and processed into the form needed for the vaccine.
Production of vaccines is often outsourced to a number of laboratories or vaccine plants around the world, and the techniques used need to be standardised.
Quality control is problematic in some parts of the world.
In some cases preservatives are added to vaccines, and some require constant refrigeration - a problem in hot countries with intermittent power supplies.
Similar but different
The term
inoculation is sometimes used alongside vaccination. In fact it is the introduction of a living organism (and I think it is OK to include viruses here) into a new environment in order for it to grow and reproduce. We inoculate bacteria onto agar to grow them in Petri dishes, or into nutrient broth in tubes.
So inoculation really refers to the transfer of whole (living?) organisms, not just selected antigens from them.
Herd immunity
(community immunity)
When a sufficiently large proportion of a population has been vaccinated against a communicable disease, a situation exists whereby spread of the disease is prevented or greatly reduced. This is because even though the pathogen is released from infected individuals, there is less contact with other unprotected individuals.
This is known as
herd immunity.
Some human health workers prefer the term
community immunity which emphasises the commitment to public well-being, although it is rather a mouthful.
Case in point: Measles
Measles is a highly contagious virus disease. Although it has been considered to be a low-grade rash and fever, in about 1 case in 15 it may cause ear infections and neurological damage or pneumonia, and other conditions.
In high income regions of the world such as Western Europe, measles causes death in about 1 in 5000 cases, but as many as 1 in 100 will die in the poorest regions of the world. Worldwide, it is still a significant cause of death.
In the UK between 1940 and 1968, notified cases of measles averaged at 400,000 per year (dipping 6 times below 200,000, and reaching 600,000 5 times), and about 100 deaths per year were reported.
After the introduction of the measles vaccine in 1968, the number of cases fell in stages, and levelled out under 20,000 from 1990 onwards. This was replaced by the MMR Measles Mumps and Rubella vaccine which gives cover against 3 viral diseases, and the NHS recommends vaccination of children at about one year old (or earlier if required), followed by a second booster (before starting school?) at about 3 years of age.
It has been stated that
herd immunity is achieved with a 96% MMR vaccine coverage.
In 1998 Andrew Wakefield published a paper (now 'retracted') linking the MMR vaccine to autism and enterocolitis. By that year the number of cases of measles recorded in the UK had dropped to 56, but public reaction caused vaccination rates to fall from 90%+ to 80% in 2003/4 (and larger drops in some areas), and this resulted in a number of measles outbreaks (1370 reported cases in 2008, over 2000 in 2012).
Additionally there are a number of young people who have not had the second booster vaccination so they are not fully protected.
The NHS states that children up to the age of 18 who missed, or only partially completed, their earlier MMR vaccination can have a "catch-up" MMR vaccination on the NHS.
Several large-scale investigations failed to confirm links between MMR vaccine and autism.
In 2010 Wakefield's licence to practise as a doctor was revoked by the UK General Medical Council. They found him guilty of dishonesty, the "abuse" of developmentally delayed children by giving them unnecessary and invasive medical procedures, and acting without ethical approval for his research.
He has moved to America and continues to campaign on autism.
A similar situation has occurred in other parts of the world, but this has been exacerbated by global travel and pockets of unvaccinated people of different ethnic groups.
See web links below
There are controversial anti-vaccination campaigns which feature prominently on social media ....
Active and passive immunity
Active immunity is immunological protection against foreign organisms as a consequence of exposure to them or their antigens, as explained above. The production of antibodies takes some time to take effect, but once established it lasts for a long time - all life long in some cases.
Passive immunity is the transfer of antibodies produced in another organism and its effect is generally short-lived.
Mothers and babies
Antibodies which have developed (over time) in the blood of a pregnant female can be passed
across the placenta to the blood of the developing foetus so that it has acquired a certain amount of immunity to pathogens it may encounter after birth.
Antibodies can also possibly be passed via breast milk to the new-born baby. Colostrum, the thicker initial secretion from the mammary glands, contains a number of ingredients that assist development of new-born babies.
Antivenoms against snake bites etc
If an animal e.g. a horse or a sheep is injected with (small, but then increased) doses of snake venom, it will develop antibodies against it. Antibodies can be extracted from the blood and purified for use as a treatment for humans bitten by the same or similar species of snake. This may be called antivenin or venom antiserum.
A similar situation exists with scorpion stings, spider bites and stings from certain fishes.
Small pocks - big success
Note the past tense
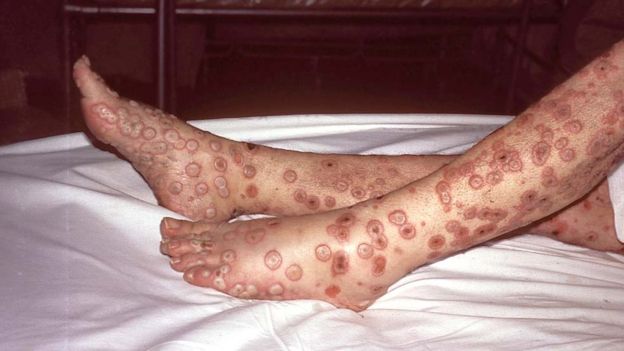
Smallpox was a very contagious disease caused by a virus - often called
variola. Symptoms included fever followed by rash on the tongue, spreading to the face and all over the body, with sunken sores ('pocks') containing an opaque fluid, eventually forming scabs which fell off. It is said that it was fatal in 30% of cases, and easily passed from person to person. It was known about three thousand years ago, spreading along trade routes between Africa, Asia and Europe, reaching the Americas in the sixteenth century.
Variolation was a rather random technique that involved giving someone a limited exposure to the virus, through material from scabs of infected people being sniffed up into the nose, or placed under the cut skin. This caused a less severe form of the smallpox (which could still spread and cause an epidemic), and usually gave immunity to those so treated. However, about 1-2% of treated people died (which is better than 30%).
By 1700, variolation was fairly commonplace in Africa, India and the Ottoman Empire, and it was introduced to England in the 1720's.
Some key names
Edward Jenner (1749-1823)
was a doctor in Berkeley, Gloucestershire. Apparently he underwent variolation as a schoolchild, and that impaired his health somewhat. As a doctor, he performed variolation on his patients. Living in the country, he knew about cowpox, a disease of cows, showing as pocks on the udder, and he heard that people who caught it from their cows could not subsequently catch smallpox.
Sarah Nelmes
was a milkmaid who had a rash on her hand, which Jenner diagnosed as cowpox.
Blossom
was the cow who passed it on to Sarah as she was being milked (by hand).
James Phipps
was the eight-year old son of Jenner's gardener, and Jenner transferred fluid from one of Sarah's pocks to 'scratches' on James' skin. He became poorly but quickly recovered. One and a half months later Jenner subjected James to variolation with smallpox material, but he did not become infected and remained immune to smallpox for the rest of his life.
Jenner published his results and went on to develop his findings and supply cowpox material (which was in fairly short supply).
He encountered opposition from other medics and other sceptical people, but he was honoured and recompensed in a number of ways.
Vaccinia
is the name given to cowpox, based on the Latin
vacca for cow. This gives its name to vaccination.
The use of cowpox vaccine gradually received acceptability to the public. In fact variolation was forbidden by Act of Parliament in 1840 and vaccination with cowpox was made compulsory in 1853.
Janet Parker
was the last person to die of smallpox, in 1978, astonishingly in the UK.
She was a medical photographer at the Birmingham University Medical School and she worked one floor above the Medical Microbiology Department where smallpox research was being conducted. As a consequence, work on smallpox around the world was dramatically scaled down.
Finishing off this disease
Following vaccination campaigns, smallpox was considered to be eliminated in North America in 1952 and Europe 1953, leaving South America, Asia, and Africa.
In 1959 the World Health Organization (WHO) launched a campaign to eradicate smallpox worldwide but they had to re-launch it in 1980. At that time they estimated that there were still up to 15 million cases of smallpox each year. Teams of vaccinators from all over the world journeyed to the remotest of communities to vaccinate every person in the areas at risk. In 1980 it was officially declared that smallpox had been eradicated. It is the only worldwide disease that has been eliminated so far.
All stocks of smallpox virus have been destroyed, with the exception of material kept under high security in the USA (CDC, Atlanta) and Russia (State Research Center of Virology and Biotechnology) in Koltsovo.
Less of a pest
In 2010, the Food and Agriculture Organization announced that the viral disease
rinderpest (in the same group as measles), which infected cattle and other ruminants had been completely eradicated, by the use of a live attenuated vaccine, making this the first (and so far the only) disease of livestock to have been eradicated.
More candidates
Measles and rubella are seen as possible candidates for eradication by continued vaccination programmes.
The risk of
polio has been greatly reduced in many parts of the world.
Its incidence has decreased by 99.9 percent; from an estimated 350,000 cases in 1988 to a low of 483 cases in 2001, after which it remained at a level of about 1,000 - 2000 cases per year for a number of years. In 2015, cases decreased to 98 and further decreased in 2016 to 37 wild cases and 5 circulating vaccine-derived cases, but increased in 2018 to 33 wild cases and 103 circulating vaccine-derived cases.
Polio is currently the subject of a global eradication program. Another is Dracunculiasis, also called Guinea-worm disease, a parasitic infection by the Guinea worm.
Thicker than water?
In the ABO series of blood groups, there are 4 types of cells.
There are 3 possible different antigens on the surfaces of red blood cells, and 3 possible antibodies in the blood plasma.
| Blood group | A | B | AB | O |
| Antigen(s) | A
| B | A and B | "none"
- but see below |
| Antibodies | anti-B | anti-A | none | anti-A and anti-B |
Antigen oligosaccharide chains
on red blood cell membranes

|

|

|
Both of these antigens are found on each AB red blood cell's membrane


|

The basic (precursor) oligosaccharide chain
|
Genes and enzymes involved
I stands for
isohaemagglutinogen
The A allele (
IA) encodes
1-3-N-acetyl galactosaminyl transferase that bonds α-N-acetyl galactosamine to the D-galactose end of the H antigen
The B allele (
IB) encodes
1-3-galactosyl transferase that bonds α-D-galactose to the D-galactose end of the H antigen
The O allele (
IO or
i) contains a nucleotide deletion that results in a loss of enzymatic activity


 MHC class II receptor
MHC class II receptor














 Red blood cells (7.5 µm) are about 60 times larger than HIV particles (120nm)
Red blood cells (7.5 µm) are about 60 times larger than HIV particles (120nm)


 Click on image above for animation
Click on image above for animation

 Azidothymidine (AZT), also known as Zidovudine (ZDV) is a thymidine analogue, causing termination of the DNA chain as it forms from viral RNA.
This has been widely used medically to prevent mother-to-child spread during birth or after other potential exposure such as a needlestick injury.
Azidothymidine (AZT), also known as Zidovudine (ZDV) is a thymidine analogue, causing termination of the DNA chain as it forms from viral RNA.
This has been widely used medically to prevent mother-to-child spread during birth or after other potential exposure such as a needlestick injury.
 Pregnancy test kits use monoclonal antibodies.
Pregnancy test kits use monoclonal antibodies.






