Glycolysis
Glycolysis is the first stage of respiration. In the cell it takes place in the cytoplasm (also known as cytosol), i.e. not inside mitochondria.
It does not require oxygen, so it may be described as anaerobic.
Glycolysis means splitting of glucose, a 6-carbon sugar, to produce pyruvate, a 3-carbon compound.
The main reaction stages are as follows:
Glucose is phosphorylated by ATP, producing glucose phosphate.
Glucose phosphate undergoes molecular reorganisation and further phosphorylation by ATP, producing fructose bisphosphate.
Fructose bisphosphate splits into 2 molecules of triose phosphate.
Triose phosphate becomes oxidised and accepts inorganic phosphate, producing bisphosphoglycerate and reduced NAD.
Bisphosphoglycerate produces monophosphoglycerate and ATP
Monophosphoglycerate undergoes molecular reorganisation producing pyruvate and ATP.
There is a net gain of 2 ATPs and 2 reduced NADs in the process. No CO
2 is given off.
Subsequent stages can take quite different directions, but there are not different versions of glycolysis.
Glycolysis stages - inside the cell

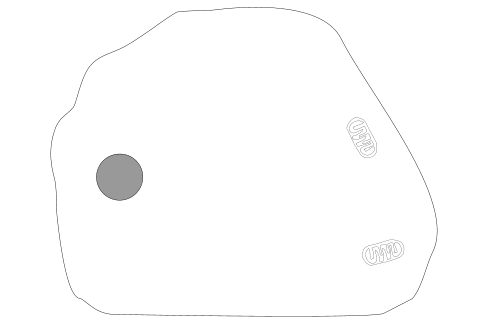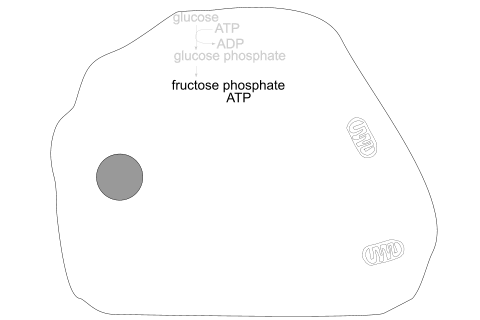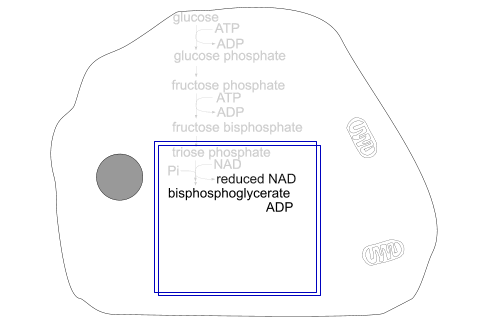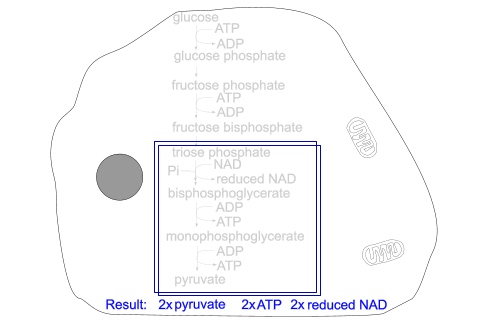
The section enclosed in blue boxes takes place twice, as 2 molecules of triose phosphate [3C] are obtained from one glucose molecule [6C] at the start.

Glycolysis in more detail
Glycolysis takes place in a series of 10 reactions. Each is controlled by its own
enzyme, and the
product of each takes part in the next reaction.

[1] The first reaction involves activating glucose with the input of energy in the form of ATP - a phosphorylation reaction.
This produces
glucose (6-)phosphate, and the enzyme involved is called
hexokinase or
glucokinase (kinase implies getting things moving).
This has the effect of stimulating the intake of more glucose into the cell via glucose transporters (GLUTs) which are proteins embedded in cell membranes.
This part is bypassed when glycogen is being broken down in muscles and liver:
glycogen phosphorylase phosphorylates glucose residues as it releases them from the tips of polyglucose branches in the glycogen molecule.

[2] This G6P is converted into
fructose 6-phosphate (F6P), (another 6-carbon, 1 phosphate sugar), by the enzyme
glucose phosphate isomerase (G6P and F6P are isomers).

[3] F6P is converted into F1,6BP (
fructose 1,6 bis-phosphate - meaning fructose with 2 separate phosphate groups, at different ends of the molecule).
The enzyme involved is
phosphofructokinase (PFK), and the second phosphate group also comes from ATP, which then becomes ADP. Incidentally, ADP has 2 phosphate groups and ATP has 3, attached to the same part of the molecule.
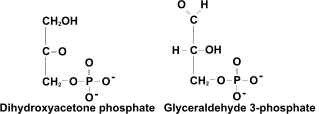
[4] Then F1,6BP (6-carbon, 2 phosphate sugar) is broken down, using the enzyme
aldolase, into two molecules of
triose phosphate TP (3-carbon, 1 phosphate sugar). In fact these are slightly different: one is glyceraldehyde 3-phosphate, and the other is dihydroxyacetone phosphate DHAP - isomers of one another.
[5]
Triose phosphate isomerase converts dihydroxyacetone phosphate into glyceraldehyde 3-phosphate so each of the next stages take place twice.
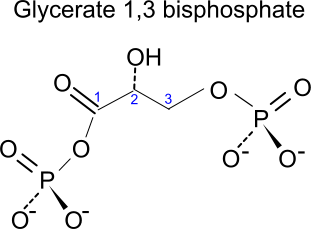
[6] The two triose phosphates then each accept another phosphate group - this time from
inorganic phosphate Pi - and become oxidised, under the action of the enzyme
triose phosphate dehydrogenase.
This forms 1
,3-bisphosphoglycerate: G 1,3 BP (conjugate base of 1,3-bisphosphoglyceric acid: 3-carbon, 2 phosphate).
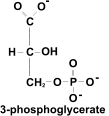
[7] G 1,3 BP gives up one phosphate group, producing
G3P and releasing (2x) ATP as a result.
[8,9] G3P is converted into other 3 carbon, 1 phosphate compounds;
G2P,
PEP.
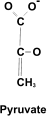
[10] Under the influence of
pyruvate kinase, PEP gives up the last phosphate group, producing
pyruvate, a 3 carbon compound, and releasing (2x) ATP as a result.



 [1] The first reaction involves activating glucose with the input of energy in the form of ATP - a phosphorylation reaction.
This produces glucose (6-)phosphate, and the enzyme involved is called hexokinase or glucokinase (kinase implies getting things moving).
This has the effect of stimulating the intake of more glucose into the cell via glucose transporters (GLUTs) which are proteins embedded in cell membranes.
[1] The first reaction involves activating glucose with the input of energy in the form of ATP - a phosphorylation reaction.
This produces glucose (6-)phosphate, and the enzyme involved is called hexokinase or glucokinase (kinase implies getting things moving).
This has the effect of stimulating the intake of more glucose into the cell via glucose transporters (GLUTs) which are proteins embedded in cell membranes.
 [2] This G6P is converted into fructose 6-phosphate (F6P), (another 6-carbon, 1 phosphate sugar), by the enzyme glucose phosphate isomerase (G6P and F6P are isomers).
[2] This G6P is converted into fructose 6-phosphate (F6P), (another 6-carbon, 1 phosphate sugar), by the enzyme glucose phosphate isomerase (G6P and F6P are isomers).
 [3] F6P is converted into F1,6BP (fructose 1,6 bis-phosphate - meaning fructose with 2 separate phosphate groups, at different ends of the molecule).
The enzyme involved is phosphofructokinase (PFK), and the second phosphate group also comes from ATP, which then becomes ADP. Incidentally, ADP has 2 phosphate groups and ATP has 3, attached to the same part of the molecule.
[3] F6P is converted into F1,6BP (fructose 1,6 bis-phosphate - meaning fructose with 2 separate phosphate groups, at different ends of the molecule).
The enzyme involved is phosphofructokinase (PFK), and the second phosphate group also comes from ATP, which then becomes ADP. Incidentally, ADP has 2 phosphate groups and ATP has 3, attached to the same part of the molecule.
 [4] Then F1,6BP (6-carbon, 2 phosphate sugar) is broken down, using the enzyme aldolase, into two molecules of triose phosphate TP (3-carbon, 1 phosphate sugar). In fact these are slightly different: one is glyceraldehyde 3-phosphate, and the other is dihydroxyacetone phosphate DHAP - isomers of one another.
[4] Then F1,6BP (6-carbon, 2 phosphate sugar) is broken down, using the enzyme aldolase, into two molecules of triose phosphate TP (3-carbon, 1 phosphate sugar). In fact these are slightly different: one is glyceraldehyde 3-phosphate, and the other is dihydroxyacetone phosphate DHAP - isomers of one another.
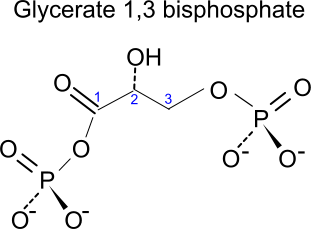

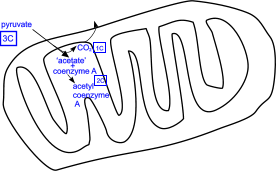
 peddled the citric acid cycle from 1937 and he was awarded the
Nobel Prize in Physiology or Medicine 1953
peddled the citric acid cycle from 1937 and he was awarded the
Nobel Prize in Physiology or Medicine 1953